Branched chain amino acids, or BCAAs for short, are small building-blocks of protein and refer to a group of 3 essential amino acids; leucine, isoleucine, and valine.
BCAAs are defined according to their unique branched chain like molecular structure and have many purported benefits, such as aiding in muscle growth.
This article presents science based evidence regarding the benefits of branched chain amino acids, as well as explaining the difference between BCAAs and essential amino acid (EAAs) supplements.
It also answers popular questions such as:
- What do BCAA supplements do?
- Do BCAA really work?
- When should I take BCAAs?
- What are the side effects of BCAA?
But that's not all, you will additionally learn how amino acids supplements are manufactured, including why vegan and fermented plant-based amino acids, like Amino-XS, are superior to standard BCAA and EAAs.
What are the benefits of BCAA, and are there any risks taking them?
BCAAs have various ergogenic benefits, here are 4 of them:
BCAAs exhibit anabolic activities by invoking muscle protein synthesis (MPS)
The anabolic effects of BCAAs,and in particular leucine, are well-documented. In fact various studies confirm the same; leucine triggers the mTOR (mammalian target of rapamycin) pathway. mTOR is a myogenic pathway that signals (MPS) muscle protein synthesis (1, 2).
In lay terms, the activation of mTOR sparks off the biological process for construction of new muscle proteins including satellite cell proliferation.
It is suggested that a minimum dose of 1.8 grams of Leucine is required, in order to trigger mTOR activation (3).
Muscle hypertrophy is governed by a combination of BCAA and overall protein intake
As the owner of a supplement company it would be all too convenient to only talk about the correlation between BCAA, mTOR activation, and muscle protein synthesis, but that wouldn't be the whole truth.
Despite Leucine’s efficacy in triggering mTOR and driving muscle protein synthesis (MPS), muscle hypertrophy is only achieved through a positive nitrogen balance; when protein assimilation supersedes protein degradation.
Skeletal muscle mass is always in a dynamic transitional state, undulating in an ebb and flow of protein anabolism versus catabolism.
As it turns out, one of the primary rate-limiting factors of nitrogen retention and muscle growth is protein intake; inadequate protein consumption results in a negative nitrogen balance, and vice versa (4).
Thus triggering mTOR and protein synthesis, although advantageous, is only part of the equation in achieving skeletal muscle hypertrophy. Overall protein intake is fundamental for muscle growth, along with having a sufficient amount of EAAs, which are also noted to be a rate-limiting factor of muscle protein synthesis (MPS).
Therefore relying on BCAAs alone to trigger mTOR is inefficient for building new muscle if overall daily protein intake is insufficient.
Nevertheless, eating a sufficient amount of protein per day, supplemented with an ample dose of pre or intra workout BCAAs, is efficacous at triggering muscle protein synthesis, thereby promoting muscle growth and hypertrophy.
BCAAs are anti-catabolic and help protect against muscle loss
Branched chain amino acids are unique not only in structure but also in the fact that they can be directly oxidised within skeletal muscle cells (5).
Furthermore BCAA’s account for the highest percentage of amino acid content in muscle proteins, and therefore comprise more mass of skeletal muscle than any other amino acids.
Exercise causes skeletal muscles to release significantly large amounts of the non-essential amino acids, glutamine and alanine. The amount released is dependent on various factors, such as exercise intensity and duration.
Nontheless, glutamine and alanine loss can soon exceed amounts available in the amino acid pool, thus leading to a deficit of these two amino acids. To compensate for this loss, the body catabolises muscle proteins to release the essential branch chained amino acids, leucine, isoleucine and valine.
These BCAAs are then used as a substrate to synthesise the non-essential amino acids, glutamine and alanine, and thus restore the balance (6).
This gives rise to BCAAs having a distinct benefit over and above all other proteinogenic amino acids.
Despite being anabolic, one of the main benefits of using a BCAA supplement is protection from exercise-driven muscle catabolism during periods of intense and/or prolongued exercise.
BCAAs help reduce the delayed onset of muscle soreness (DOMS)
Resistance training, especially eccentric exercise, is associated with muscle damage and the delayed onset of muscle soreness.
Because BCAAs trigger the mTOR pathway, it is proposed that dietary supplementation of BCAAs, taken either pre, during, or post exercise, could help speed recovery and thus reduce exercise-induced muscle soreness.
One study in particular carried out by the International Society of Sports Nutrition (ISSN) gives credence to this proposition. The ISSN tested the effects of BCAA supplementation on exercise-induced muscle damage in resistance trained men.
The results confirmed the same, with the researchers concluding:
“The present study has shown that BCAA administered before and following damaging resistance exercise reduces indices of muscle damage and accelerates recovery in resistance-trained males.”
BCAAs may help reduce central fatigue
Experiencing fatigue from exercise is pretty much inevitable, especially if you tend to push yourself hard. Reduction in tensile output and exercise-performance of skeletal muscles, is partly due to impairments in the neuro-muscular system.
Because skeleto-muscular cross-bridge formations and contractions are initiated in the brain, central fatigue may occur when changes within the central nervous system (CNS) reduce the capacity of signalling to the neuro-muscular junction.
Peripheral or central fatigue is associated with elevated cerebral levels of the amino acid tryptophan (7).
Tryptophan is the precursor of the neurotransmitter serotonin (5-hydroxytryptamine (5-HT)), a factor that has been observed to increase in humans experiencing fatigue while exercising.
Large neutral amino acids (LNAA) transport
The branched chain amino acids leucine, isoleucine and valine are part of the large neutral group of amino acids, which are able to pass through the blood brain barrier and enter the brain.
When BCAAs are present with other large neutral amino acids, such as tryptophan, each one enters into contention with the other for transport across the blood brain barrier.
This results in less of each amino acid crossing the barrier and entering the brain (8).
During exercise, especially resistance training, skeletal muscles rapidly utilise BCAAs causing an overall decrease in circulatory BCAA concention (9). A reduction in BCAAs enables more tryptophan to pass through the blood brain barrier.
Since tryptophan is a rate-limiting factor of 5-hydroxytryptamine (5-HT) synthesis, supplementing with BCAAs during exercise, can help reduce uptake of tryptophan into the brain and thus lower serotonin, which is proposed to delay the onset of central fatigue (10).
Amino-XS® BCAA And EAA Intra Workout Drink 448 Grams

£29.99
£31.99
DNA lean® Amino-XS® is a state of the art BCAA & EAA intra workout drink specifically designed to optimise athletic performance, stimulate muscle growth, and promote recovery from intense workouts. If you need that extra push with your workouts and… Read More
LEARN MORE > Be limitless: An Ultimate Guide To Nootropics (NEW RESEARCH)
BCAA risks and side effects
BCAAs are essential nutrients, that is, the human body cannot endogenously synthesis them and therefore can only be obtained through dietary sources. As a general rule, BCAAs are safe to consume.
It is counter intuitive to think that essential nutrients have an associated health risk.
BCAAs are also contained in lots of protein rich foods, and if BCAAs did have an associated health risk, this would also mean that foods containing substantial amounts of BCAAs could potentially be unsafe.
However, because BCAA supplements are highly concentrated, over scooping can easily result in an unordinary high level of BCAA that could not be achieved from eating protein rich foods alone.
According to McCance and Widdowson the Composition of Foods, 100 grams of raw chicken breast contains:
Isoleucine: 1.44 grams
Leucine: 2.34 grams
Valine: 1.5 grams
Total BCAAs: 5.28 grams
Therefore, half a kilogram of chicken breast (500 grams) yields approximately:
Isoleucine: 7.2 grams
Leucine: 11.7 grams
Valine: 7.5 grams
Total BCAAs: 26.4 grams
For most folk, consuming a massive half a kilogram of chicken breast per day might be impractical.
However, assuming a serving size of 13 grams, just 2 scoops per day delivers approximately the same volume of BCAAs as half a kilogram of chicken breast.
That said, due to the concentrated nature of BCAA powders and capsules, it may be possible to consume too much BCAAs, of which the effects are not well-documented.
As a general rule of thumb, 1-2 scoops of BCAAs taken on training days (pre, intra and post workout) should be ample for any athlete.
After an intense workout, BCAAs and other amino acids help to repair and build new muscle proteins.
BCAA requirements and dosage
Although government health agencies recommend figures for men and women regarding daily protein intake, protein requirements can significantly vary from individual to individual.
For instance, Public Health England recommend a minimum daily protein intake of 55.5 grams and 45.0 grams, for men and women respectively.
This is no where near enough for athletes, bodybuilders, and folk who are generally active. For instance, The International Society of Sports Nutrition recommend 1.4 - 2.0 grams of protein per kilogram of body weight, per day, for individuals who frequently engage in physical exercise.
However, individual amino acid requirements are less well-documented. Figures from other studies using non-trained humans also significantly vary. For instance 2 separate studies had a combined value of all 3 BCAAs (leucine, isolecine & valine) ranging from 84mg per kilogram of bodyweight to 210mg per kilogram of bodyweight (11, 12).
For a 90 kilogram human, these figures would equate to a daily BCAA intake between 7.6 grams - 18.9 grams.
For athletes, protein and thus amino acid requirements are much greater than sedentary folk. However as of yet the jury is still out on individual amino acid requirements.
Foods high in branched chain amino acids (BCAAs)
Contrary to popular belief, lots of plant-based foods contain both BCAAs, as well as the other additional essential amino acids (EAAs). However, generally speaking, plant-based foods do not contain BCAAs and essential amino acids (EEAs) in the volumes as animal based foods do.
Although chicken was covered earlier, here are addituonal 4 foods that are relatively high in leucine, isoleucine and valine (BCAA content per 100 grams).
1. Whey protein powder
Leucine 8.6 grams
Isoleucine 3.8 grams
Valine 3.5 grams
Total BCAA = 15.9 grams
2. Soybeans
Leucine 5.0 grams
Isoleucine 1.9 grams
Valine 2.2 grams
Total BCAA = 9.1 grams
3. Haddock
Leucine 1.9 grams
Isoleucine 1.0 grams
Valine 1.2 grams
Total BCAA = 4.2 grams
4. Quark (soft cheese)
Isoleucine 0.6 grams
Leucine 0.9 grams
Lysine 0.8 grams
Total BCAA = 2.3 grams
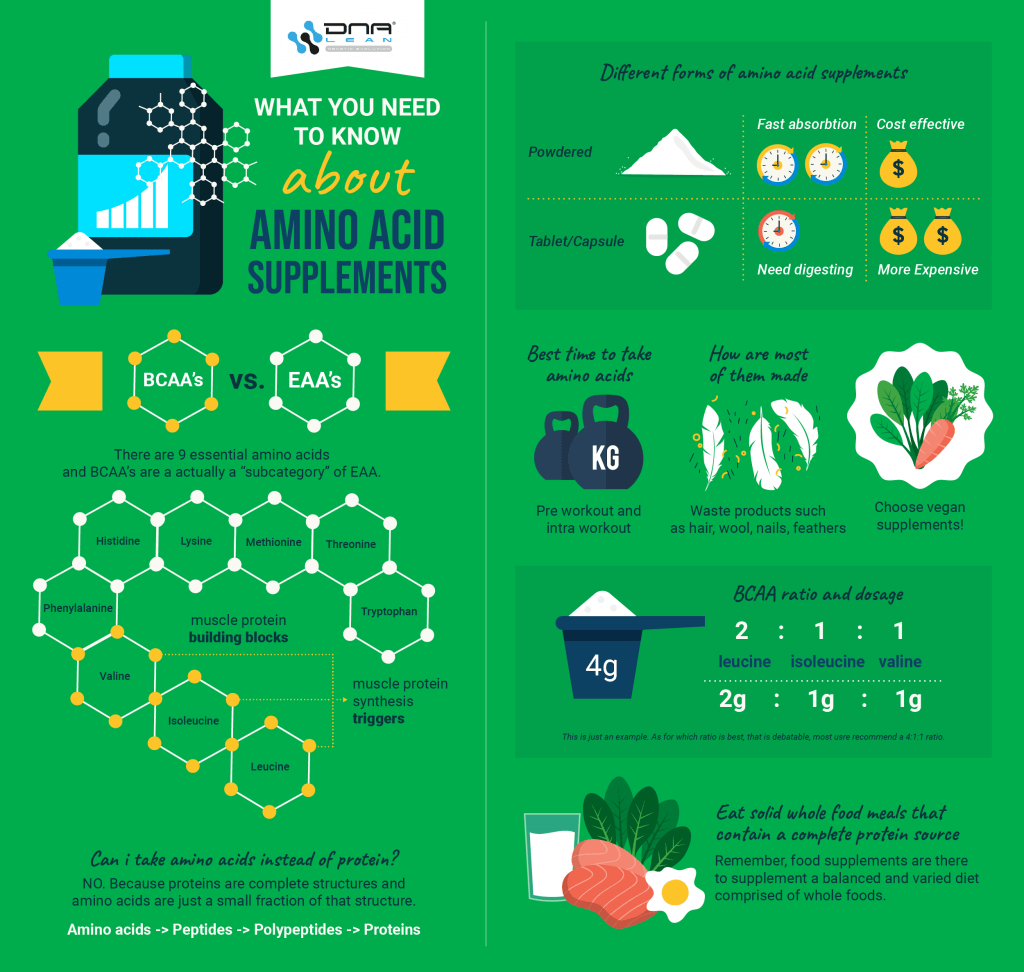
What is the difference between BCAA and EAA supplements?
On occasion I hear people talk controversially about BCAA’s. Some claim BCAA’s are a complete waste of money, some swear by them – while other athletes say they prefer EAA’s.
Ironically, BCAA’s are EAA’s.
Branched chain amino acids are in fact essential amino acids. The terms BCAA and EAA are largely misunderstood.
The acronym EAA stands for essential amino acid. This simply refers to an amino acid that your body cannot synthesise from a combination of other amino acids. Thus it is essential because it needs to be obtained through dietary means.
There are 9 essential amino acids, these are:
- Leucine (BCAA)
- Isoleucine (BCAA)
- Valine (BCAA)
- Histidine
- Lysine
- Methionine
- Phenylalanine
- Threonine
- Tryptophan
BCAA’s are a “subcategory” of EAA. Leucine, Valine and Isoleucine are all essential branched chain amino acids. These three amino acids although essential, are primarily referred to as BCAA’s because of their “branched chain” molecular structure which is distinctly different from the other 6 essential amino acids.
Other amino acids (non-essential & conditionally essential)
BCAA’s and EAA’s aren’t the full story. Classification of amino acids goes further than just these two groups. The remaining proteinogenic amino acids are classed as either non-essential or conditionally essential.
Non-essential amino acids
- Alanine
- Aspartic acid
- Asparagine
- Glutamic acid
- Serine
Non-essential amino acids are as you would expect the opposite of essential amino acids. Needless to say the non-essential amino’s can be synthesised from a combination other amino acids and do not specifically need to be obtained from food.
If the body is short on any non-essential amino’s, it can manufacture them. However, this may slow down protein synthesis.
It is therefore optimal to consume a variety of protein sources, that contain a variety of amino acids, to ensure protein synthesis is always fully and properly facilitated.
Conditionally essential amino acids (and why they are important)
- Arginine
- Cysteine
- Glycine
- Glutamine
- Proline
- Tyrosine
Conditionally essential amino acids are a relatively new classification and although these amino acids can be manufactured by the body, under certain pathophysiological conditions their manufacture can become limited. Thus they are termed conditionally essential.
What are the different forms of BCAAs and amino acid supplements?
Amino acids are available in either free form or peptide bonded versions. Free form amino acids are unbound to any other amino acids and are in their singular free form rather than being incorporated into a peptide sequence. You can think of free form amino acids as individual lego bricks.
Peptide bonded amino acids are small chains of amino acids that are bound together; Pre-digested proteins if you like. You can think of amino acid peptide molecules as blocks of many lego bricks.
Both free form and peptide bonded amino acid supplements are available either in a ready to mix powder or a tablet/capsule version. There are two main differences between the powdered and tablet/capsule versions.
- Tablets and capsules require dispersal in the stomach before the BCAAs and amino acids are released, and therefore take slightly longer to enter the body. If you need a quick fix, opt for the powdered versions.
- Tablets and capsules work out considerably less cost effective. The price per gram is usually significantly higher than their powdered counterpart.
BCAA vs. EAA supplements - which are better for building muscle?

Simply put, BCAA’s, and particularly leucine, serve as a modulator of protein synthesis, as well as being proteinogenic.
Nevertheless muscle protein synthesis can only occur when all 9 essential amino acids are present.
This finding has recently been made basis for the claim that BCAA’s on their own are pointless, and that a full compliment of EAAs are more important for building muscle.
I mean, why bother signalling mTOR and muscle protein synthesis (MPS), if there isn't the full compliment of essential amino acids to facilitate protein anabolism?
Actually it’s not so hard and fast so don’t get ready to write off your BCAA’s just yet.
Consider this - unless you fail to consume a pre-workout meal, that includes a complete protein source, it is unlikely that there would be an unavailibility of the other essential amino acids that facilitate protein synthesis.
Therefore, neither BCAAs nor EAAs are best for building muscle. Rather, the two work together in synergy to stimulate and facilitate muscle protein synthesis.
BCAAs have an additional benefit over and above EAAs due to their anti-catabolic properties and ability to be oxidised directly by skeletal muscle tissues.
So why compromise your hard work at the gym by not supplementing with BCAA’s?
It is no coincidence that top athletes and bodybuilders alike, use a mixture of both essential and branch chained amino acids during training, and so should you. Take advantage of them.
LEARN MORE > Pre Workout Nutrition: 6 Of The Best Pre Workout Meals You Need To Know
EAAs facilitate protein synthesis (muscle building)
If you want to conquer muscle building, essential amino acids are an absolute MUST. Remember, the human body cannot synthesise any essential amino acids whatsoever. These powerful building blocks of muscle have to be obtained through dietary sources.
If you fall short on just one essential amino acid, or even if they are all present, but only in meager amounts, the effect is catastrophic for muscle building; protein synthesis is be brought to a grinding halt.
Therefore EAA’s are the backbone of building muscle and if they become unavailable, muscle proteins cannot be assimilated. That said, it is also important to remember that the term essential amino acids, also refers to branched chain amino acids; BCAA’s are absolutely essential.
Collectively, these amino acids (BCAA + EAA) are required to enable muscle protein anabolism, leading to hypertrophy. Therefore, combining both together and in optimum ratios, can yield astounding results.
Leucine along with isoleucine and valine kick-start protein synthesis. This muscle building process is then facilitated by an adequate complement of essential amino acids. Bam – your fast-track to muscle growth and hypertrophy.
What is the BCAA ratio?
BCAA supplement labels usually display a BCAA ratio. This is generally either a 2:1:1 ratio, or a 4:1:1 ratio. Nevertheless, other ratios are also available. For instance, I have even seen a 10:1:1 ratio.
The BCAA ratio simply refers to the ratio of leucine to isoleucine and valine, based on weight. For example a 4 gram serving of BCAA’s in a 2:1:1 ratio would yield 2 grams of leucine and a gram each of isoleucine and valine.
The ratio of leucine is always higher because it is primarily leucine that triggers the mTOR pathway. In order to achieve a dose of 2.5 grams of leucine, using a 2:1:1 ratio, a serving size of 5 grams of BCAA’s is required.
A 5 gram serving of 2:1:1 BCAAs also yields 1.25 grams of both isoleucine and valine.
As for which ratio is best, this is debatable and I am not aware of any study that gives the definitive answer to this. That said, I personally shoot for a 4:1:1 ratio. Although I am basing this on anecdotal evidence, I feel that this ratio has always provided the best results for my clients and I.
What sources are BCAA and EAA supplements derived from?
One of the most shocking facts that has recently headlined the sports supplements industry, is where amino acid supplements are commonly derived from.
Picture this - when you’re next at the gym sipping on your refreshing drink of BCAA’s, you could well be drinking a mix of hair soup.
Waste products such as hair, wool, nails, feathers etc, all contain a protein called keratin. These waste products are then processed using toxic chemicals that break apart the proteins and eventually extract the constituent amino acids which are then sold as amino acid supplements.
You've probably seen ads on TV for keratin based shampoo, the reason for including keratin in shampoos is because it supports healthy and strong hair.
This is why dna lean insist on using premium vegan friendly plant-based fermented amino acids in their Amino-XS supplement.
Natural fermentation yields a cleaner and superior chemical free product. Furthermore, using plant protein sources achieves piece of mind, knowing that you are not ingesting amino acids from some old chicken feathers.
When is the best time to take BCAA and amino acids?
Amino acid supplements can literally be taken any time of day; nevertheless the best time to take amino acids (both BCAA’s and EAA’s) is pre workout and intra workout.
Taking amino acid supplements either pre or intra workout is 100% guaranteed to give you better results.
If you take advantage of this magic window of opportunity you will immediately notice a shift in your workouts; a mediocre workout will become epic.
This is because amino acids and peptides are pre-digested, they quickly pass through the stomach and enter into the bloodstream where they are rapidly delivered straight in to your muscles cells through a process known as hyperemia (13).
Amino acid supplements can peak blood levels within 30 minutes, however as fast as blood levels can peak, they can also decline. This is why it is important to continuously sip on your BCAAs during training to maintain a steady and constant supply.
The best part, using amino acid supplements during training will promote faster recovery along with an increase in muscle growth.
This is partly due to the anti-catabolic effect of branched chain amino acids, but if you opt to supplement with a BCAA/EAA combo, such as Amino-XS , you can also boost protein synthesis.
When should you choose BCAA’s and EAA’s over whey protein?
As I previously mentioned, exercise (especially resistance training) promotes the catabolism of muscle protein BCAA stores. Additionally, ingesting a large dose of amino acids while sedentary, can lead to oxidation by the liver (a process where amino acids are broken down and used as energy, producing urea as a by-product).
Oxidation of amino acid supplements is wasteful (not to mention expensive) and results in EAAs and BCAAs missing their proteinogenic function.
Regarding whey protein, whey has an impressive amino acid profile which is super high in both essential and branched chain amino acids. In fact whey protein has one of the most complete amino acid profiles, in respect of human nutrition, of any protein source whatsoever.
Nevertheless whey protein is an intact protein and requires digestion. It cannot be absorbed as quickly as, nor does it provide amino acids as readily as consuming free form amino acids.
It is important to pay careful consideration as to when you opt to consume either a protein supplement or amino acid supplement. Needless to say you want to receive their full and optimum effect.
Recommendations: How to best take BCAAs and amino acid supplements
Based on the evidence set out earlier in this article, it is strongly recommend supplementing with BCAAs and amino acids either pre workout or intra workout. This is because pre or intra workout BCAAs:
1. Are used as a substrate for glutamine and alanine synthesis and thus protect against muscle catabolism during exercise.
2. Trigger mTOR to stimulate muscle protein synthesis.
Conversely, with whey protein, it is recommend to consume it post workout for optimum results. This helps to aid recovery and to further prolong protein synthesis that was earlier triggered by the branced chain amino acid leucine.
Take home thoughts
Any other time of day simply eat solid whole food meals that contain a complete protein source that contains all 9 of the essential amino acids. Remember, food supplements are there to supplement a balanced and varied diet comprised of whole foods.
More on amino acids: the building blocks of life
Simply put, amino acids are the most fundamental building-blocks of life. Amino acids in various different sequences and ratios collectively form proteins of which make up all life as we know it, whether it is a single cell amoeba or a human being made of trillions of cells.
Furthermore, amino acids have a myriad of other functions, and aside from being proteinogenic (protein creating), they also participate in a number of other important biological processes that are essential to our existence.
For example, there are countless other non-proteinogenic amino acids that partake in various other activities, such as; enzymatic function, neurotransmitter function, immune system function, and the biosynthesis of hormones (14, 15).
What is role of amino acids in your body?
One of the major functions of protenogenic amino acids is to regulate protein synthesis by signaling myogenic pathways (16, 17).
Protein synthesis is a complex process involving the transcription of new proteins with the help of messenger and transfer RNA. The end result is that protein structures are built from free amino acids.
Amino acids form the proteins that are in turn incorporated into your body. Protein structures form cells and cells then form your organs, skin, hair and bones etc.
This process of synthesising new protein structures is balanced out by the loss of proteins through cellular degredation.
The balance of newly constructed proteins vs protein degredation, is known as your nitrogen balance, which can be either negative, equilibrium, or postive states.
What is the difference between amino acids and proteins?
It is true that proteins are made from amino acids, yet the two are distinctively different.
The most basic sequences of amino acids are known as peptides which are short chains of amino acid monomers linked together using amide bonds (commonly referred to as peptide bonds).
The shortest peptide is a dipeptide which is only 2 amino acids in length. Peptides can significantly vary in length.
For example dipeptides are superseded by tripeptides (a chain of 3 amino acids). Chains of amino acids as peptides sequentially go all the way up in size to become oligopeptides (10-20 amino acids) and polypeptides.
A polypeptide is a single linear chain of many amino acids, although usually consisting of 50 amino acids or less.
It is important to note that peptides are not proteins. In fact the word “peptide” has its roots in Greece, descending from the word; peptós, meaning “digested”.
To summarise amino acid and protein structure sequences:
Amino acids -> Peptides -> oligopeptides -> Polypeptides -> Proteins
Proteins also have different levels of structure and there are in fact 4 different types of protein structure ascending is complexity:
- Primary structure
- Secondary structure
- Tertiary structure
- Quaternary structure
Relative to amino acids, proteins are huge 3d structures that form the fundamental structure of all cells within your body (not just skeletal muscle). Tissues are made up individual cells, cells contain proteins and proteins are made from amino acids.
The human body can be likened to one huge jigsaw puzzle all pieced together as one functional working unit.
Can I take amino acids instead of protein?
You can, but this is not recommended.
Although there are various benefits of taking amino acid supplements, amino acids are not complete structures like proteins are. Amino acids should not be used in place of protein.
Even if you supplemented with all 9 essential amino acids, you could not properly replace a first class protein source.
This is because
- Amino acids are readily oxidised
- Amino acids are not complete structures
- Amino acids are too rapidly metabolised to sustain themselves in the blood
Furthermore, human muscle proteins contain a full complement of 20 proteinogenic amino acids, not just 9 essential amino acids.
And finally...
A brief insight into the biochemistry of amino acids (bonus section)
Amino acids are carbon based molecules that contain both an amine (-NH2) and carboxyl acid (-COOH) functional group; hence their name title; amino acid.
Various different elements of the 118 known elements of the periodic table can be found in the side chains of amino acids. However the key constituents of all amino acids are carbon (C) hydrogen (H) nitrogen (N) and oxygen (O).
It is estimated that there are around 500 naturally occurring amino acids, although in the human genetic code there are only 20. Different sequences of these 20 proteinogenic (protein producing) amino acids make up all proteins coded by human DNA.
Further to these 20 amino acids, there are an additional two extremely rare amino acids. These are known as the 21st and 22nd amino acids respectively; Selenocysteine (Sec) and pyrrolysine (Pyl).
Although these two amino acids are proteinogenic, there is much debate as to whether these two amino acids are part the human genetic code.
Cool fact: In chemistry amino acids are known to have a hydrophobic property. Simply put, this means that amino acids repel water, consequently, amino acids are largely insoluble in water.
Have you ever mixed up a serving of BCAA’s in a shaker bottle and noticed that the powder clumped together at the top? If so, you witnessed the hydrophobic effect in full force.
Does that sound familiar?
This hydrophobic effect happens because amino acid molecules have an overall non-polar electrical charge. A non-polar charge prohibits the integration of amino acids into polar water (H2O) molecules.
Fortunately technological advancements in manufacturing processes have enabled amino acids supplements to evolve, most of which are now far more water soluble.
This is why the fermented plant-based amino acids in Amino-XS dissolve surprisingly easily, and ensure maximum absorption.
Conclusion
Branched chain amino acid supplements are efficacious at stimulating muscle protein synthesis (MPS), and have a multitude of benefits. BCAAs are both anabolic & anti-catabolic, may delay the onset of central fatigue, and help reduce post-exercise muscle soreness (DOMS).
BCAA’s can be used on their own to great effect, or can taken in conjunction with other EAA’s to both stimulate and facilitate the synthesis of new muscle proteins.
Additionally, BCAAs can be further combined with other amino acids such as glutamine, creatine, beta alanine, and taurine, for even greater effect.
BCAAs and amino acids in general, are most beneficial when taken either pre, intra, or post-exercise.
Amino acids should not be used to in place of proteins. Both have their function and both are different – use them accordingly to get maximum benefit.
One last consideration, optimal gut health is crucial for amino acid absorption, actually for any nutrient absorption for that matter. The gut requires a balance of various different probiotic organisms to function correctly and to properly absorb food stuffs.
My final piece of advice - look after your gut.
So now it’s your turn, what amino acid supplement do you use and how has it helped you?
Scientific Reference Data:
- Branched-Chain Amino Acid Ingestion Stimulates Muscle Myofibrillar Protein Synthesis following Resistance Exercise in Humans
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5461297/ - Leucine-Enriched Nutrients and the Regulation of mTOR Signalling and Human Skeletal Muscle Protein Synthesis
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5096790/ - Excess Leucine Intake Enhances Muscle Anabolic Signaling but Not Net Protein Anabolism in Young Men and Women
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2955876/ - Dietary Protein and Muscle Mass: Translating Science to Application and Health Benefit
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6566799/ - Exercise Promotes BCAA Catabolism: Effects of BCAA Supplementation on Skeletal Muscle During Exercise
https://pubmed.ncbi.nlm.nih.gov/15173434/ - Relation Between Glutamine, Branched-Chain Amino Acids, and Protein Metabolism
https://pubmed.ncbi.nlm.nih.gov/11844643/ - Essential Role of Excessive Tryptophan and Its Neurometabolites in Fatigue
https://pubmed.ncbi.nlm.nih.gov/22384494/ - Large Neutral Amino Acids: Dietary Effects on Brain Neurochemistry and Function
https://pubmed.ncbi.nlm.nih.gov/22677921/ - Serotonin and central nervous system fatigue: nutritional considerations
https://academic.oup.com/ajcn/article/72/2/573S/4729641 - A Role for Branched-Chain Amino Acids in Reducing Central Fatigue
https://pubmed.ncbi.nlm.nih.gov/16424144/ - The Total Branched-Chain Amino Acid Requirement in Young Healthy Adult Men Determined by Indicator Amino Acid Oxidation by Use of L-[1-13C]phenylalanine
https://pubmed.ncbi.nlm.nih.gov/12730426/ - Branched-chain Amino Acid Requirements in Healthy Adult Human Subjects
https://pubmed.ncbi.nlm.nih.gov/16365094/ - Regulation of Increased Blood Flow (Hyperemia) to Muscles During Exercise: A Hierarchy of Competing Physiological Needs
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4551211/ - Amino Acids: Metabolism, Functions, and Nutrition
https://pubmed.ncbi.nlm.nih.gov/19301095/ - Amino Acid Neurotransmitter Transporters: Structure, Function, and Molecular Diversity
https://pubmed.ncbi.nlm.nih.gov/8102052/ - Exogenous amino acids stimulate net muscle protein synthesis in the elderly.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC508787/ - Regulation of protein synthesis by amino acids in muscle of neonates
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3139365/















 USD
USD
 EUR
EUR